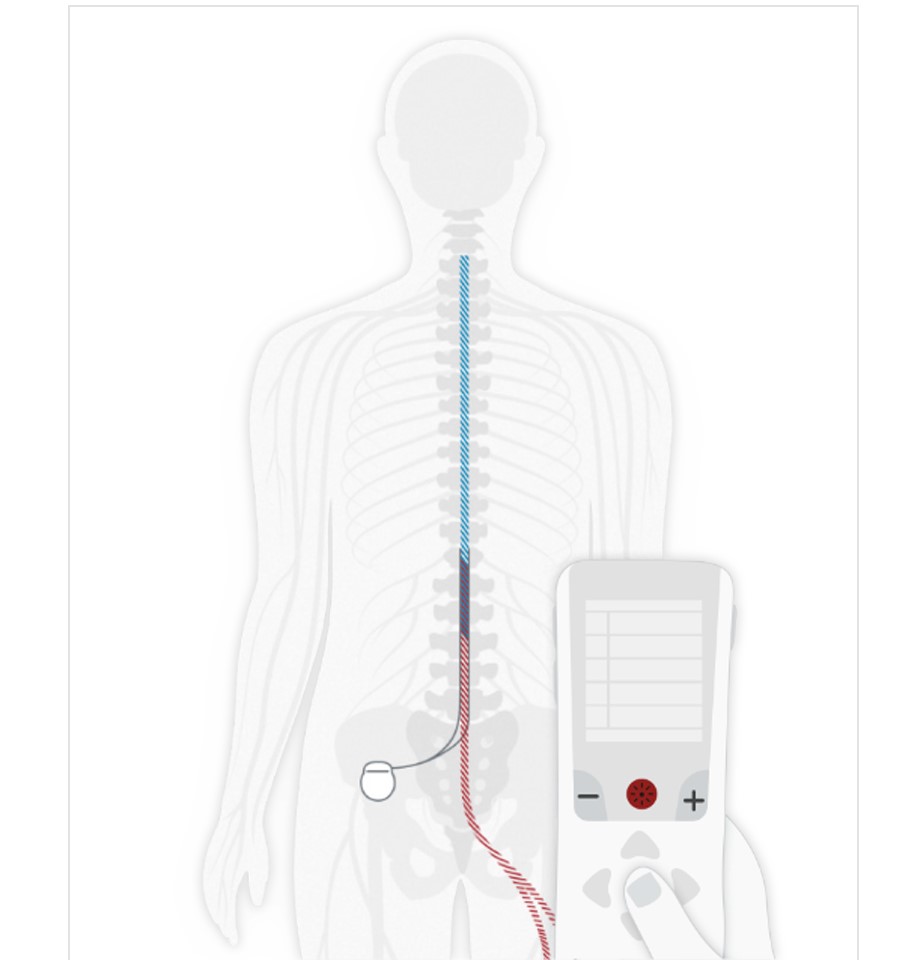

Stimulator or Implantable Pulse Generator (IPG)
A small device called a spinal cord stimulator or IPG is implanted under the skin.

Leads
Thin, flexible “leads” are connected to the spinal cord stimulator and placed near specific nerves in the back.

SCS Impulses
The spinal cord stimulator or IPG sends mild pulses through the leads to the nerves.

Pain Signals
The pulses interrupt the pain signals on the way to the brain.

Remote Control
You can turn the device on and off and adjust programs. Rechargeable systems will also include a charger.
Initially, your doctor will conduct a thorough medical evaluation. This includes a detailed history of your pain, previous treatments and any underlying health conditions.
You will likely need imaging tests such as MRI or CT scans to help visualize the spine and assess any structural issues that may be contributing to your pain. These tests provide your healthcare team with essential information to determine if SCS is appropriate for your specific situation.
A psychological evaluation is crucial to identify mental health issues that could affect SCS treatment success. It ensures you are emotionally prepared and that SCS will help with pain management.
You’ll generally be able to drive again after recovery, but it’s recommended to turn off the stimulation before driving, as sudden changes in sensation may affect your concentration or comfort.
Swimming is allowed once your incisions have fully healed, though it’s important to confirm with your doctor. Make sure to keep any external device components, like the controller, dry and away from water.
Boston Scientific advises against the use of an SCS system in a hyperbaric chamber or while scuba diving. No specific tests have been performed to guarantee the devices will function as intended or be safe under these conditions.
Level I COMBO
Randomized Controlled Trial¹
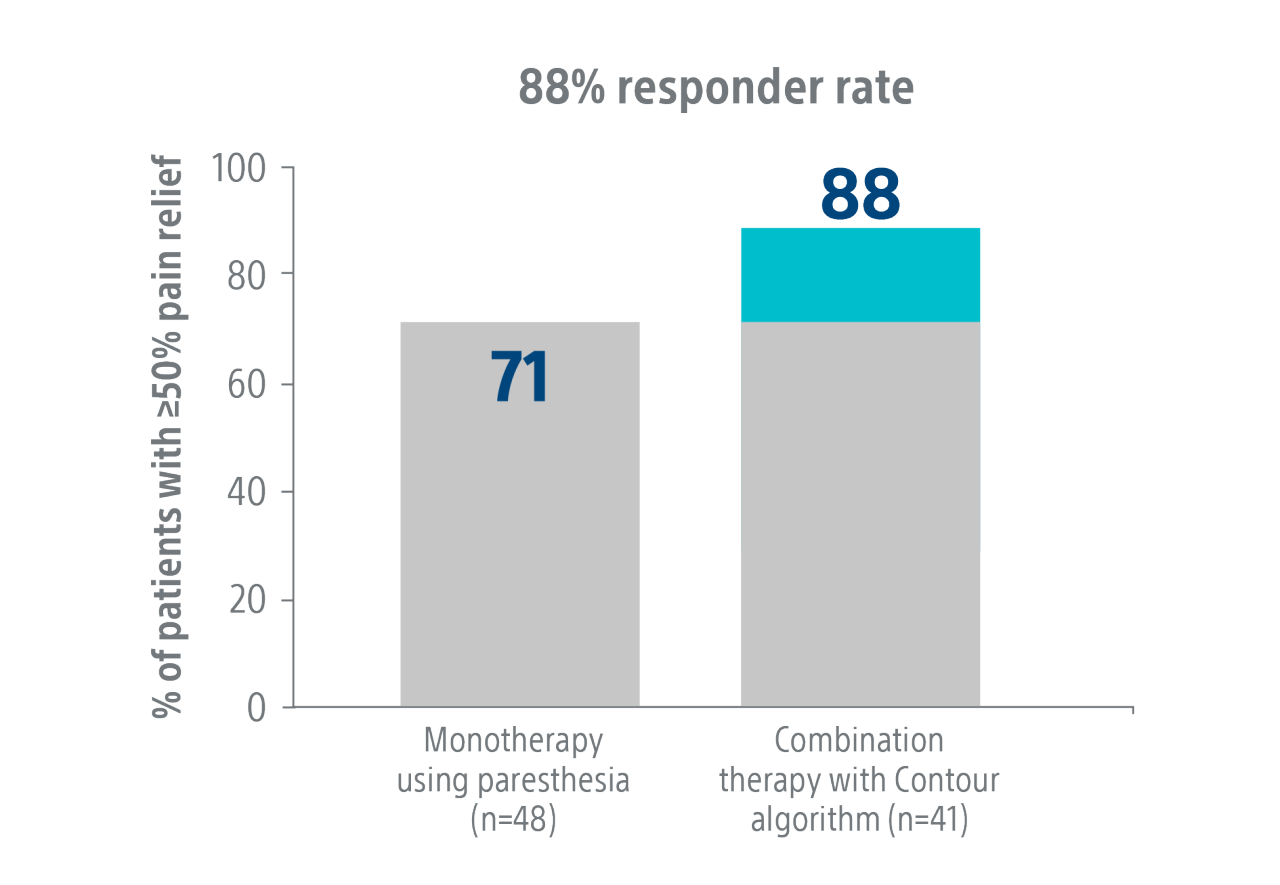
Combination Therapy using the Contour™ Algorithm achieves a higher level of clinical success.
Wavewriter
Real World Study²
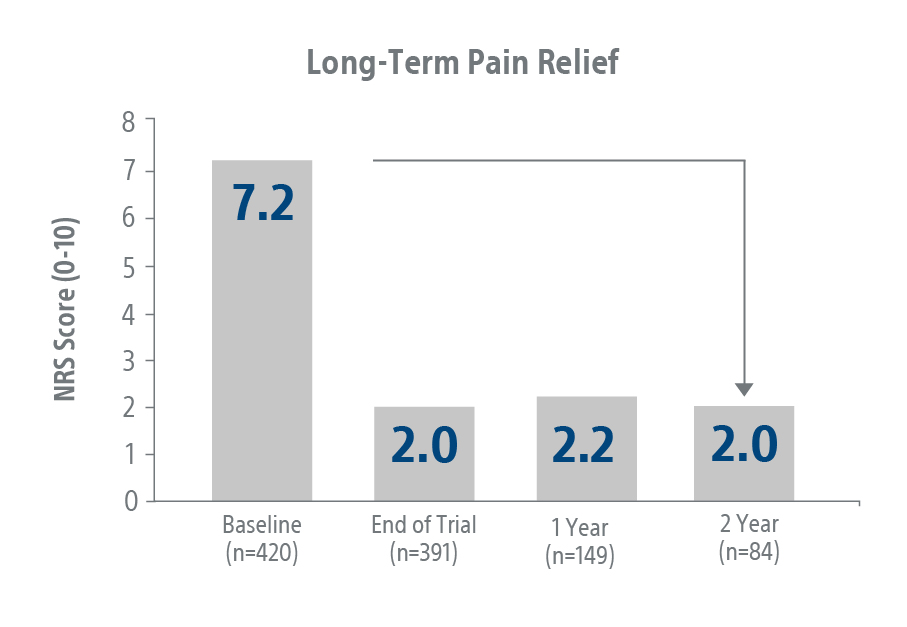
The Wavewriter™ Alpha SCS System delivers excellent durable outcomes in the real world.

References:
1. Wallace MS, North JM, Phillips GM, et al. Combination therapy with simultaneous delivery of spinal cord stimulation modalities: COMBO randomized controlled trial. Pain Manag. 2023;13(3):171-184.
2. Metzger C, Hammond B, Pyles S, et al. Three-Year Outcomes of a Large, Multicenter, Real-World Study Utilizing an SCS system with Combination Therapy. Neuromodulation: Technology at the Neural Interface 2023; 26(4):20. doi 10.1016/j.neurom.2023.04.032.
3. Stojanovic MP, Abdi S. Spinal cord stimulation. Pain Physician. 2002;5(2):156-166.
This material is for informational purposes only and not meant for medical diagnosis. This information does not constitute medical or legal advice, and Boston Scientific makes no representation regarding the medical benefits included in this information. Boston Scientific strongly recommends that you consult with your physician on all matters pertaining to your health.
CAUTION: The law restricts these devices to sale by or on the order of a physician. Indications, contraindications, warnings, and instructions for use can be found in the product labelling supplied with each device or at www.IFU-BSCI.com. Products shown for INFORMATION purposes only and may not be approved or for sale in certain countries. This material not intended for use in France.